FDA authorizes another booster dose of the Pfizer or Moderna COVID-19 vaccine for people age 50 and up - The Boston Globe
FDA greenlights Pfizer booster shot for certain groups; CDC advisory panel votes to recommend boosters | AHA News

Omicron Subvariant Now Dominant in U.S.; FDA, CDC OK Second Booster for Adults Over 50 | Hartford HealthCare | CT
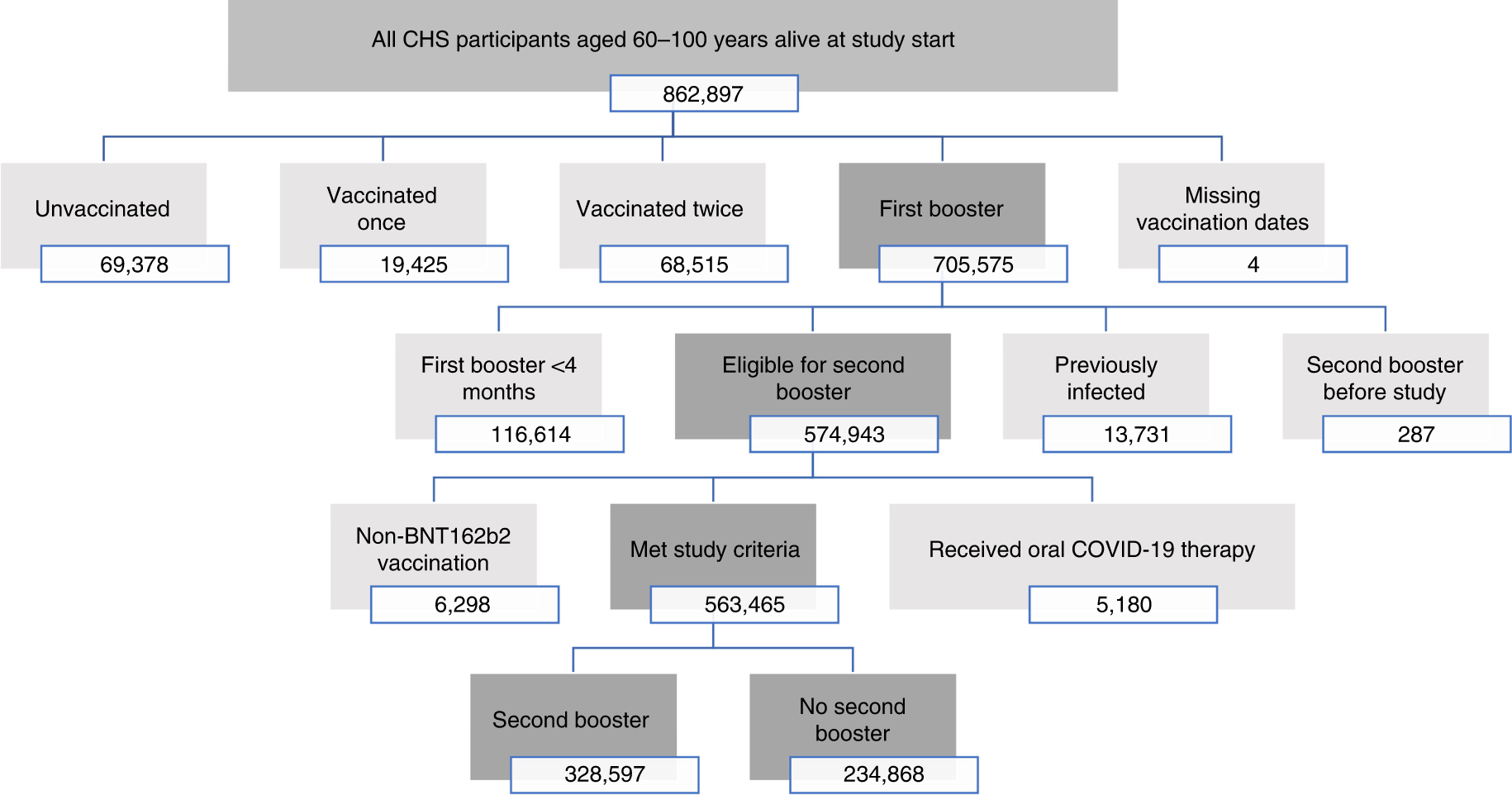
Effectiveness of a second BNT162b2 booster vaccine against hospitalization and death from COVID-19 in adults aged over 60 years | Nature Medicine

FDA authorizes second booster dose of two COVID-19 vaccines for ages 50+ and immunocompromised individuals

FDA, CDC allow second booster for adults 50+ and immunocompromised people | Northfield Hospital + Clinics