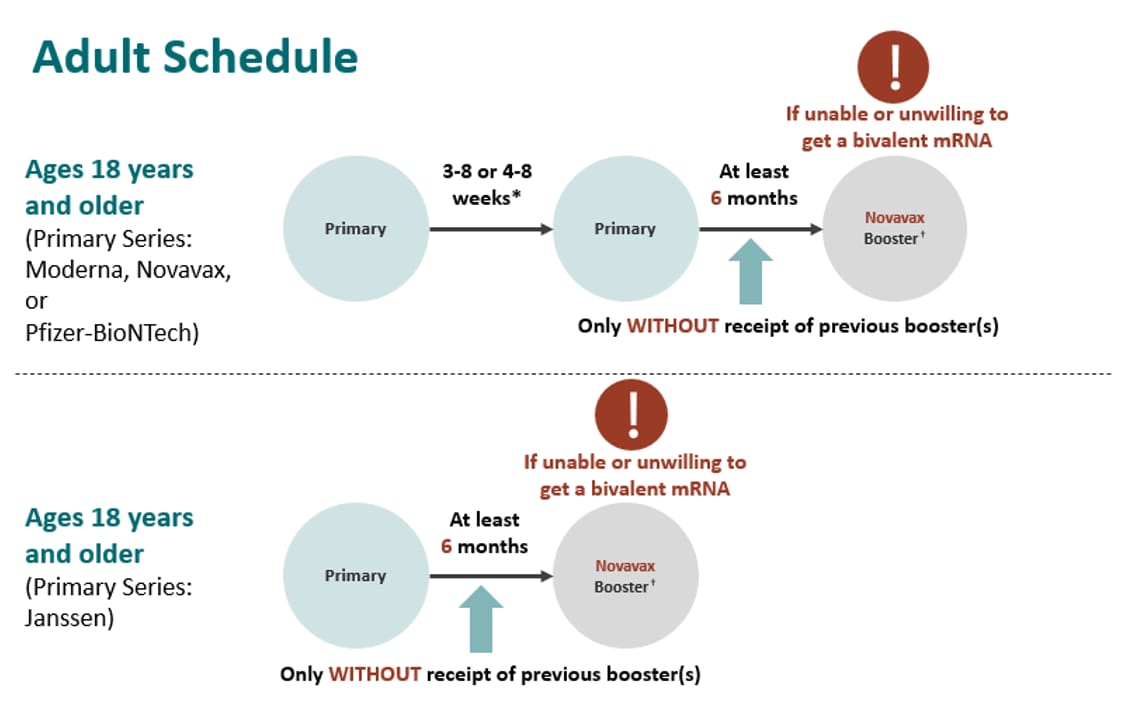
ACIP Evidence to Recommendations (EtR) for Use of Novavax COVID-19 Vaccine Booster Dose for adults ages 18 years and older under an Emergency Use Authorization | CDC
New research suggests booster dose of Novavax NVX-CoV2373 vaccine is effective against SARS-CoV-2 Omicron subvariants
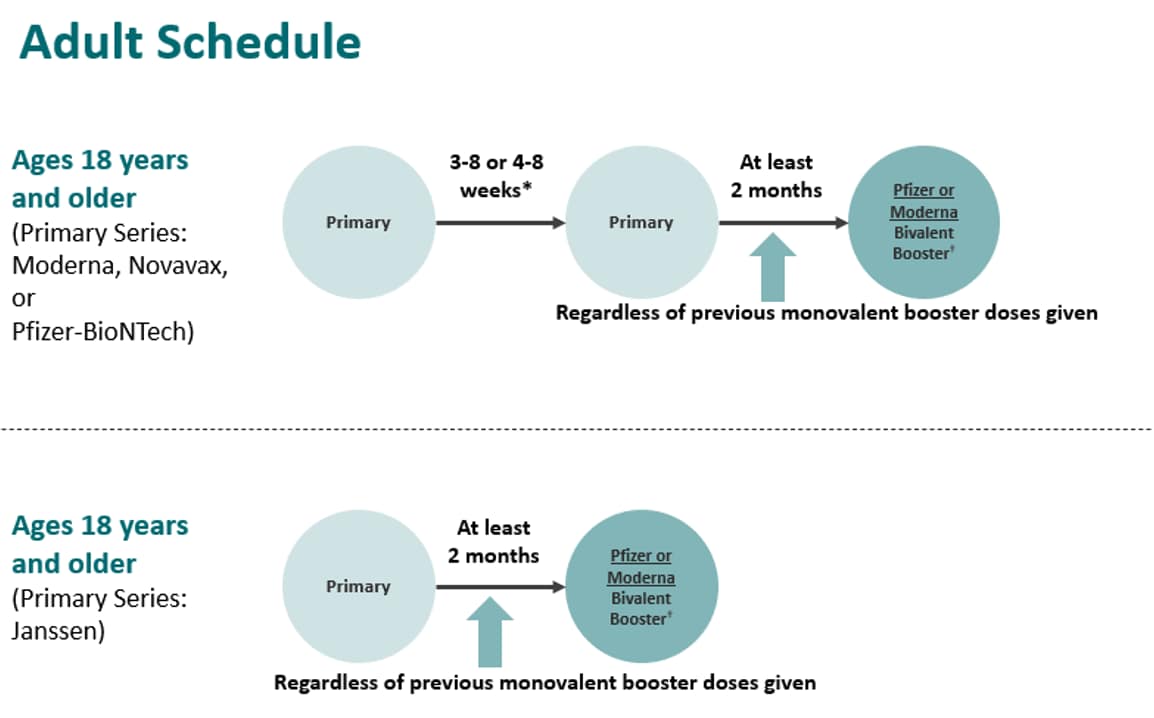
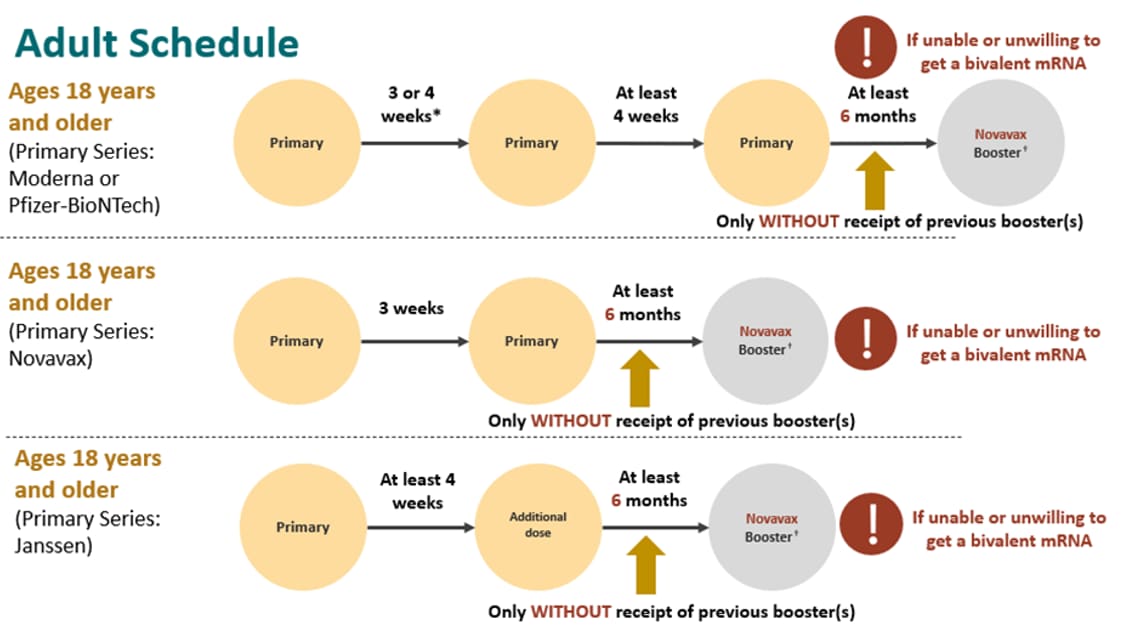
ACIP Evidence to Recommendations (EtR) for Use of Novavax COVID-19 Vaccine Booster Dose for adults ages 18 years and older under an Emergency Use Authorization | CDC

Novavax: risultati positivi per vaccino Covid come booster e per vaccino combinato Covid-Influenza – Daily Health Industry






/cloudfront-us-east-1.images.arcpublishing.com/gray/COUI7Y75YBEDLGZLB356FFMMC4.jpg)