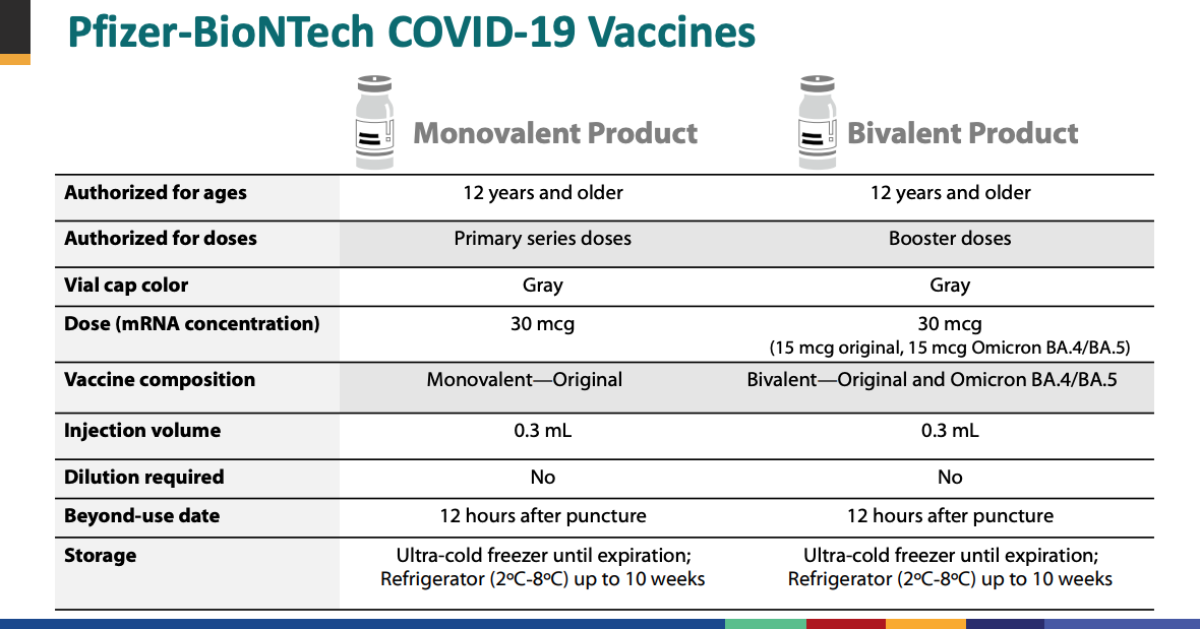
Pfizer asks FDA to authorize Covid booster shots that target omicron BA.5 for people ages 12 and older
News - CHMP Recommends Authorisation of Comirnaty Variant (BioNTech/Pfizer) Adapted to Omicron BA.4/BA.5 As Booster Vaccination for Children Aged 5 to 11 Years - Paul-Ehrlich-Institut
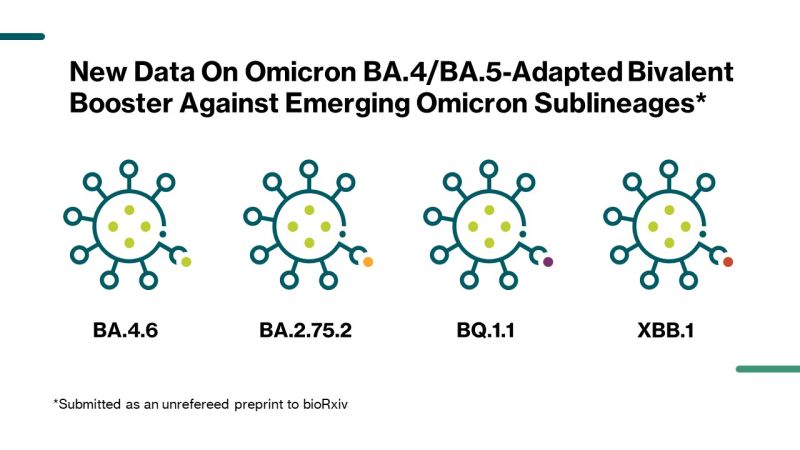
BioNTech SE on LinkedIn: Pfizer and BioNTech Report New Data on Omicron BA.4 /BA.5-Adapted Bivalent…

Pfizer Canada - Another step forward in the fight against COVID-19: Health Canada has authorized COMIRNATY Original & Omicron BA.4/BA.5 (Pfizer-BioNTech bivalent, Omicron BA.4/BA.5-adapted COVID-19 vaccine) as a booster dose for Canadians

BioNTech SE on X: "We announced today with @Pfizer updated 30-day data for the 30-µg booster dose of our Omicron BA.4/BA.5-adapted bivalent COVID-19 vaccine. https://t.co/h2ZEfBpuNb https://t.co/oaOxsYgPSk" / X

News - CHMP Recommends Additional Authorisation Modification of Comirnaty (BioNTech/Pfizer) As a Bivalent Vaccine Adapted to Omicron BA.4/BA.5 for Booster Vaccinations - Paul-Ehrlich-Institut
Antibody response from Omicron BA.4/BA.5 bivalent booster no better than original vaccine - Hospital Pharmacy EuropeHospital Pharmacy Europe
Covid: Aifa approva il vaccino bivalente Comirnaty sviluppato contro Omicron 4-5 | Sanità24 - Il Sole 24 Ore